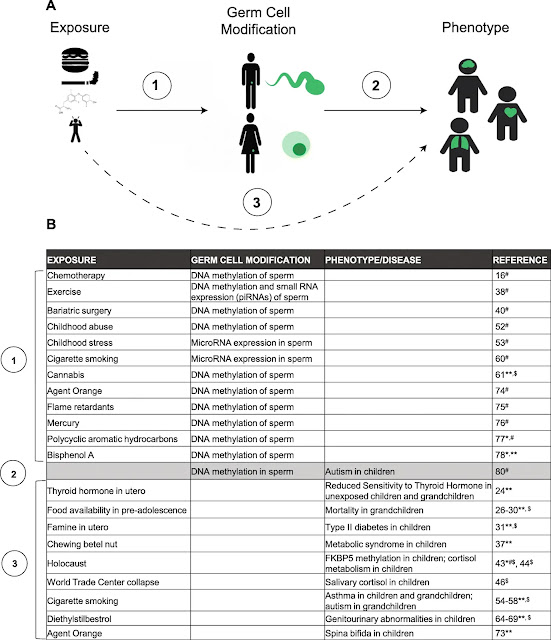
Phenotypes can be inherited in a DNA-independent manner
https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-020-00929-yExcerpt: "It is becoming increasingly apparent that certain phenotypes are inherited across generations independent of the information contained in the DNA sequence, by factors in germ cells that remain largely uncharacterized. As evidence for germline non-genetic inheritance of phenotypes and diseases continues to grow in model organisms, there are fewer reports of this phenomenon in humans, due to a variety of complications in evaluating this mechanism of inheritance in humans. This review summarizes the evidence for germline-based non-genetic inheritance in humans, as well as the significant challenges and important caveats that must be considered when evaluating this process in human populations. Most reports of this process evaluate the association of a lifetime exposure in ancestors with changes in DNA methylation or small RNA expression in germ cells, as well as the association between ancestral experiences and the inheritance of a phenotype in descendants, down to great-grandchildren in some cases. Collectively, these studies provide evidence that phenotypes can be inherited in a DNA-independent manner; the extent to which this process contributes to disease development, as well as the cellular and molecular regulation of this process, remain largely undefined.
The traditional Mendelian model of inheritance states that phenotypes are inherited based on the transmission of DNA sequences across generations, and diseases are inherited when these DNA sequences are abnormal. In model organisms, it is becoming increasingly apparent that phenotypes and disease risk can be inherited from sperm and oocytes in the absence of DNA mutations or variations. This non-genetic inheritance is based on the concept that non-DNA molecules in sperm and oocytes are inherited at fertilization and modify the phenotype of the offspring, sometimes across multiple generations. The central concept in this field is that an organism’s exposures (diet, stress, chemicals, etc.) affect the composition of germline non-DNA molecules, and in this manner, these exposures can affect phenotypes in descendants. Localization of this phenomenon to the germ cells is proven by the use of in vitro fertilization with surrogate “mothers” carrying the fetus who never experienced the exposure. While there are multiple examples of this phenomenon across a variety of model organisms, the role of this process in human inheritance is less well described, and the cellular and molecular mechanisms that drive the non-genetic inheritance of phenotypes across generations are similarly poorly characterized.This process is often referred to as “epigenetic inheritance.”
Epigenetics refers to the modification of a phenotype without a change to the DNA sequence itself, and epigenetic factors are specific molecules that affect gene expression without changing the DNA sequence; examples include DNA methylation and chromatin modifications. In sperm and oocytes, these epigenetic molecules can be inherited at fertilization and thereby affect fetal organ development by modifying patterns of gene expression. These germline epigenetic factors are potentially altered based on an individual’s experiences and exposures, and in this manner, epigenetic abnormalities in sperm and oocytes can have significant effects on a descendant’s risk of disease. The germline factors discussed here include DNA methylation and small RNAs. DNA methylation is the covalent addition of a methyl group to DNA, which often leads to gene silencing. Small RNAs are a diverse group of molecules that do not code for protein; rather they have diverse (and in some cases, poorly understood) functions including the modulation of gene expression. Small RNAs are not traditional epigenetic molecules because they mostly target RNA molecules rather than DNA, and are therefore more accurately described as post-transcriptional gene expression regulators. Examples of small RNAs include miRNA, piRNA, snoRNA, and tRNA-derived fragments. Histone modifications are another common epigenetic modification which is not discussed in this review because their role in germline-mediated inheritance is largely undescribed, due to the epigenetic reprogramming that characterizes germ cell development (see below). While epigenetic factors are important contributors to inherited phenotypes caused by ancestral exposures in model organisms, their role in human exposure-driven inheritance is undescribed; thus, we use the term “non-genetic inheritance” to describe this process in humans.
"